What is Nuclear Fission?
For an attempt of producing transuranic elements, Professor Femi and his co-workers observed that when the neutrons are being bombarded on the nucleus, the β-particles would be emitted.
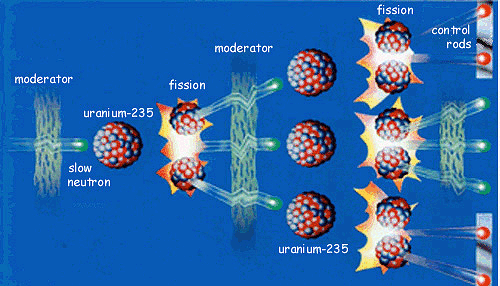
The Hahn and Strassman found out that are about 92U235 on the bombardment by the neutron producing 56Ba139, emit β-radiation, and be converted into the 57La140. Nuclear Fission is the term known for the splitting of heavy nucleus into the daughter elements. There are actually two categories you could find for the nuclear fission, the first one is the Prompt Fission and the other one is the Delayed Fission.
Whenever an atom, just like the uranium is being bombarded by high energy, which is neutron, the nucleus will then immediately split. This is a rapid type of fission. With the delayed type of fission, the bombarded neutron will be absorbed by the target nucleus or the uranium exciting the nucleus that split up later on.
With nuclear fission, the heavy nucleus will be taken when fission already has the mass of 130-149 u and the light one already has its mass of 85-104 u. Whenever the nucleus of heavy element or its isotope split up without being bombarding with the particle, the fission will be happened, it is known as the Spontaneous Fission.
Throughout the nuclear fission, some of the neutrons are being released. For instance, in 92U235 fission, on the average of 2-3, the neutrons will be released. Here is the sample that you have to consider during the fission:
92U235 + 0n1 → 35Mo95 + 57La139 + 20n1
On the left hand side, the sum of atomic masses will be
235.124 u + 1.009 u = 236.133 u
For the right hand side, the atomic masses would be:
94.946 u + 138.955 u + 2.018 u = 235.919 u
The difference in total mass of the reactants as well as products will be known as the mass defect. Its sample is:
236.133 u – 235.919 u = 0.214 u
This lost mass or the mass defect will be converted into energy. In accordance to the mass energy equation of Einstein, when 1 u mass has been converted, 931.48 MeV energy will be produced. So, with the above example, 0.214 u mass will be converted to produce 200 MeV energy, which is somewhat equivalent into the 3.2 x 10-11 Ws of the energy.
The fission of 1 Kg of this 92U235 will produce 109 W per day or 1000 MW per day energy. If there is only 30% of this energy is converted into the electrical energy of which 2500 tons of coal would be required. Moreover, the electrical energy will be produced by using up the chain reaction that the neutrons produced into past reaction being used for split uranium nuclei into the subsequent steps. This kind not fission is being carried around into special kind of vessels known as the Nuclear Reactor.